Vapor Pressure and Boiling Point:
Vapor pressure is the pressure exerted by a liquid
in equilibrium with its pure liquid phase at a given temperature.
- The vapor pressure of a liquid is dependent only upon
the nature of the liquid and the temperature.
- Different liquids at any temperature have different
vapor pressures.
- The vapor pressure of every liquid increases as the
temperature is raised.
The normal boiling point of any pure substance is the
temperature at which the vapor pressure of that substance is equal to 1
atmosphere (760 mm Hg).
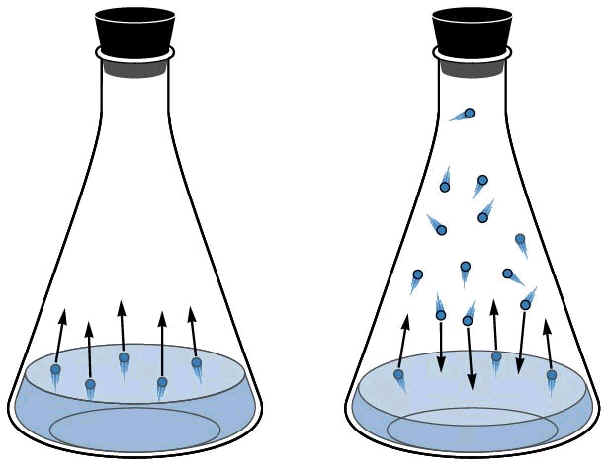
A
B
In container A, the liquid is
evaporating. Some of the molecules have enough kinetic energy to escape
(turn to a gas) by pushing against the pressure of the atmosphere.
Container B shows the flask is saturated. When new molecules of liquid are
vaporized, the gas cannot hold additional molecules, therefore some of the
molecules condense back to liquid.
Vapor
Pressure of Water at Selected Temperatures
Last modified
Contact us for more info

| 